Fujifilm Clinical Learning and Innovation Center
Welcome to the Fujifilm Clinical Learning & Innovation Center, or CLIC. The CLIC is an online space intended for endoscopy clinician use for those seeking to reference endoscopic clinical imaging innovations available in the US today. Currently, the CLIC offers a growing library of clinical educational content and resources on Image Enhanced Endoscopy (IEE) and Endoscopic Submucosal Dissection (ESD).
IEE Image Enhanced Endoscopy
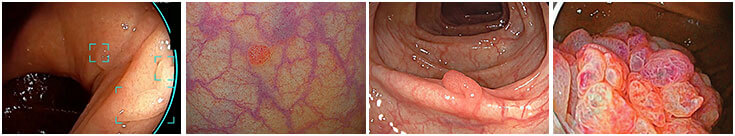
Transforming Endoscopic Capabilities.
Since the introduction of flexible gastrointestinal endoscopy, Image-Enhanced Endoscopy (IEE) has emerged as a new paradigm, playing a central role in real-time diagnosis and treatment of gastrointestinal pathologies, and radically improving disease prognosis in both upper and lower GI applications.
ESD Endoscopic Submucosal Dissection
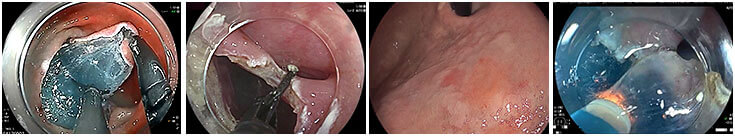
Simplifying ESD. Advancing Endoscopy.
First conceptually described in the 1990s, ESD is evolving in the US as a feasible and effective procedure for treatment of early gastrointestinal tract (GIT) neoplasia, transforming the disease management paradigm for multiple premalignant conditions and several types of early esophageal, gastric, and colon cancers.
