Fujifilm: Advancing IEE. Revolutionizing Endoscopy.
Since the introduction of flexible gastrointestinal endoscopy in the 1960s, successive technological advances — developed by multiple manufacturers — have fundamentally improved its diagnostic and therapeutic potential. Image-enhanced endoscopy (IEE) has emerged as a new paradigm, playing a central role in real-time diagnosis and treatment of gastrointestinal pathologies, and radically improving disease prognosis.
In colonoscopy, current image enhancement technologies enable lesion identification, measurement, and optical histologic diagnosis with increased accuracy compared to conventional white light endoscopy (WLE), facilitating screening and immediate decisions for definitive disease management.
Fujifilm has been a key player in both the inception and continuous evolution of IEE. FICE — the first digital post-processing technology — was introduced by Fujifilm in 2005. Today, we offer two unique light modes, BLI and LCI, delivering unique functionality and evidence-backed clinical utility.
Endoscopy, Screening, and Global Cancer Burden
Gastrointestinal (GI) cancers account for more than 25% of the global cancer incidence and over one-third of all cancer-related deaths (Fig. 1), numbers which are predicted to increase by 58% and 73%, respectively, by 2040. This pending burden highlights the need for timely implementation of preventative strategies and future clinical services planning.
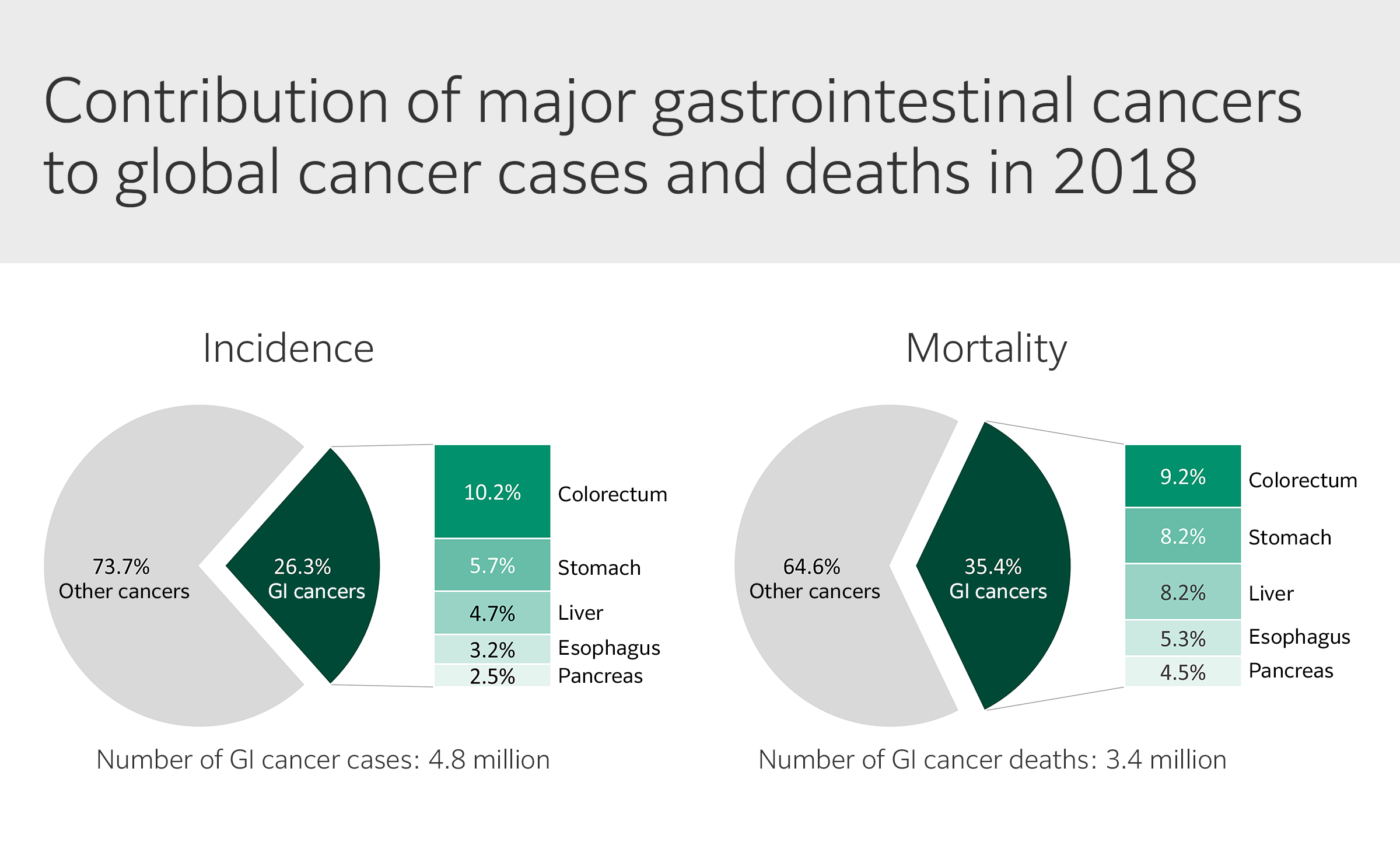
Although cancers of the stomach, liver, esophagus, pancreas, and colorectum — the primary malignant conditions of the GI tract — share common risk factors, they are distinct in etiology and epidemiologic profiles. GI cancer type distribution also varies across world regions, with higher prevalence of esophageal, gastric, and liver cancers in Asia, whereas colorectal and pancreatic cancers are more common in Europe and North America.
Upper endoscopy has an established role in diagnosing, screening, and palliation of esophageal, gastric, liver, and pancreatic cancers; however, early detection of most UGI cancers remains extremely difficult, and treatment options are limited or unavailable. Although screening programs have shown promising results in high-risk regions, the lower incidence of UGI cancers in other areas can limit opportunities for hands-on training, reducing the numbers of endoscopists with upper GI proficiency in Europe and the US. UGI cancers therefore continue to pose a major challenge to public health.
Excluding some skin cancers, colorectal cancer (CRC) is the fourth most common cancer diagnosed in men and women in the US, ranking second in cancer-related deaths overall when numbers for men and women are combined (Fig. 2). CRC mortality is directly linked to stage at diagnosis, and is highly preventable through regular screening, surveillance, and high-quality treatment. Several screening strategies are available, with colonoscopy widely recognized as the gold standard. Colonoscopy has enabled expansion of periodic screening to asymptomatic individuals, offering the single most productive opportunity to reduce the burden of disease. Today, more than one third of CRCs are diagnosed at the localized stage, with a 5-year relative survival of > 91%. Enabling both early-stage detection and removal of precancerous polyps, colonoscopy yields a 69% reduction in new cases and an 88% decrease in CRC-related deaths.
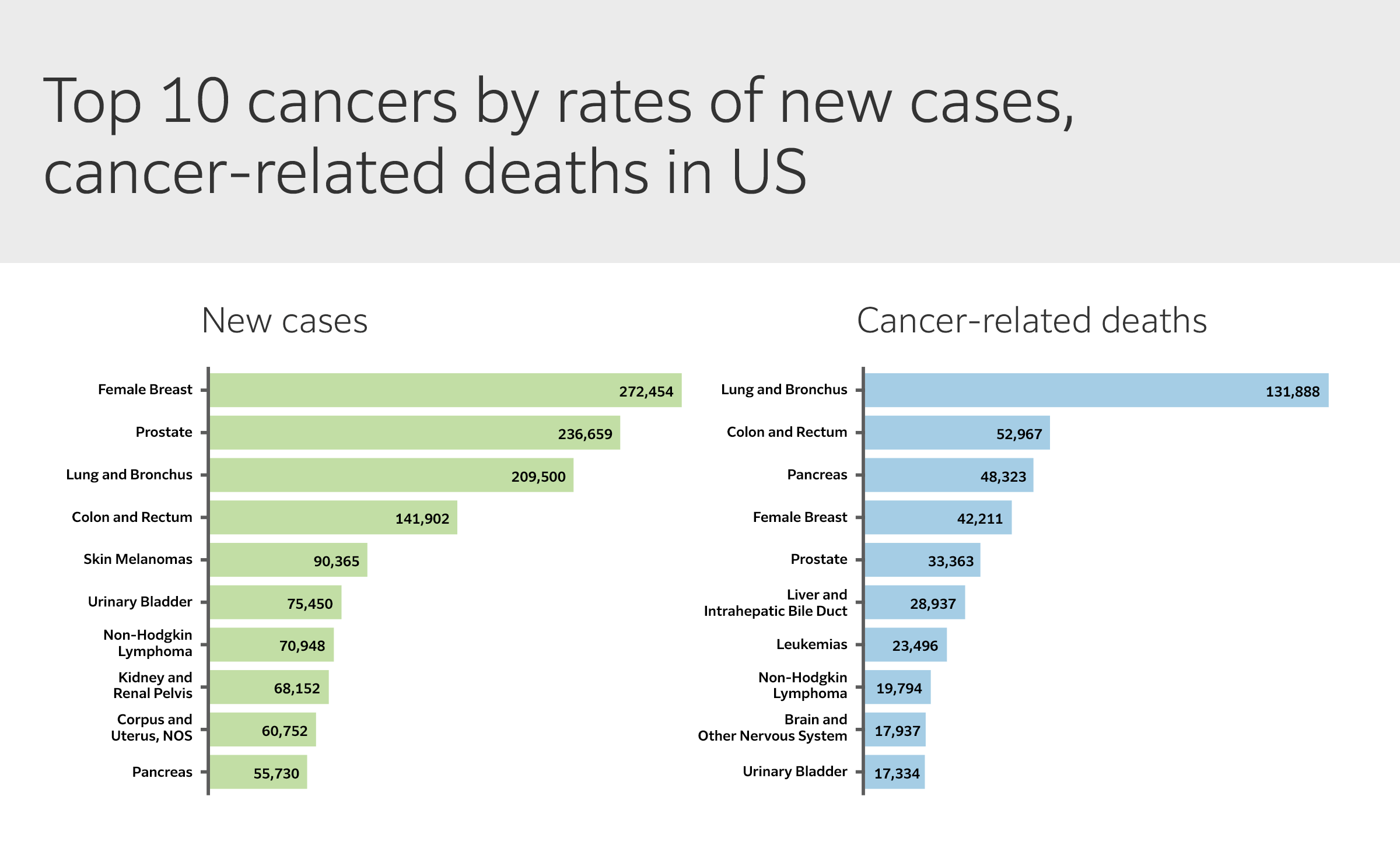
(Modified from US Cancer Statistics Data Visualizations Tool).
Despite these encouraging trends, CRC incidence continues to rise globally — particularly in younger adults — and diagnosis is occurring at more advanced stages. Updated recommendations now endorse starting screening at age 45, which is estimated to yield a 21% reduction in CRC-related deaths. With more than 15 million colonoscopies performed annually in the US for CRC screening and surveillance, this projected uptake in procedures further underscores the need to both expand the roles of colonoscopy and reduce its limitations. Colonoscopy is still a highly operator-dependent procedure, and factors like bowel preparation and lesion location, shape, and anatomy can lead to missed lesions.
Measuring Quality in Colonoscopy
Failure to detect adenomas during colonoscopy is well-documented and associated with increased risk of cancer: lesions missed are responsible for 50%–60% of interval cancers. Expertise and methodology can greatly influence colonoscopy success, an inherent variability that requires a numerical standardization framework.
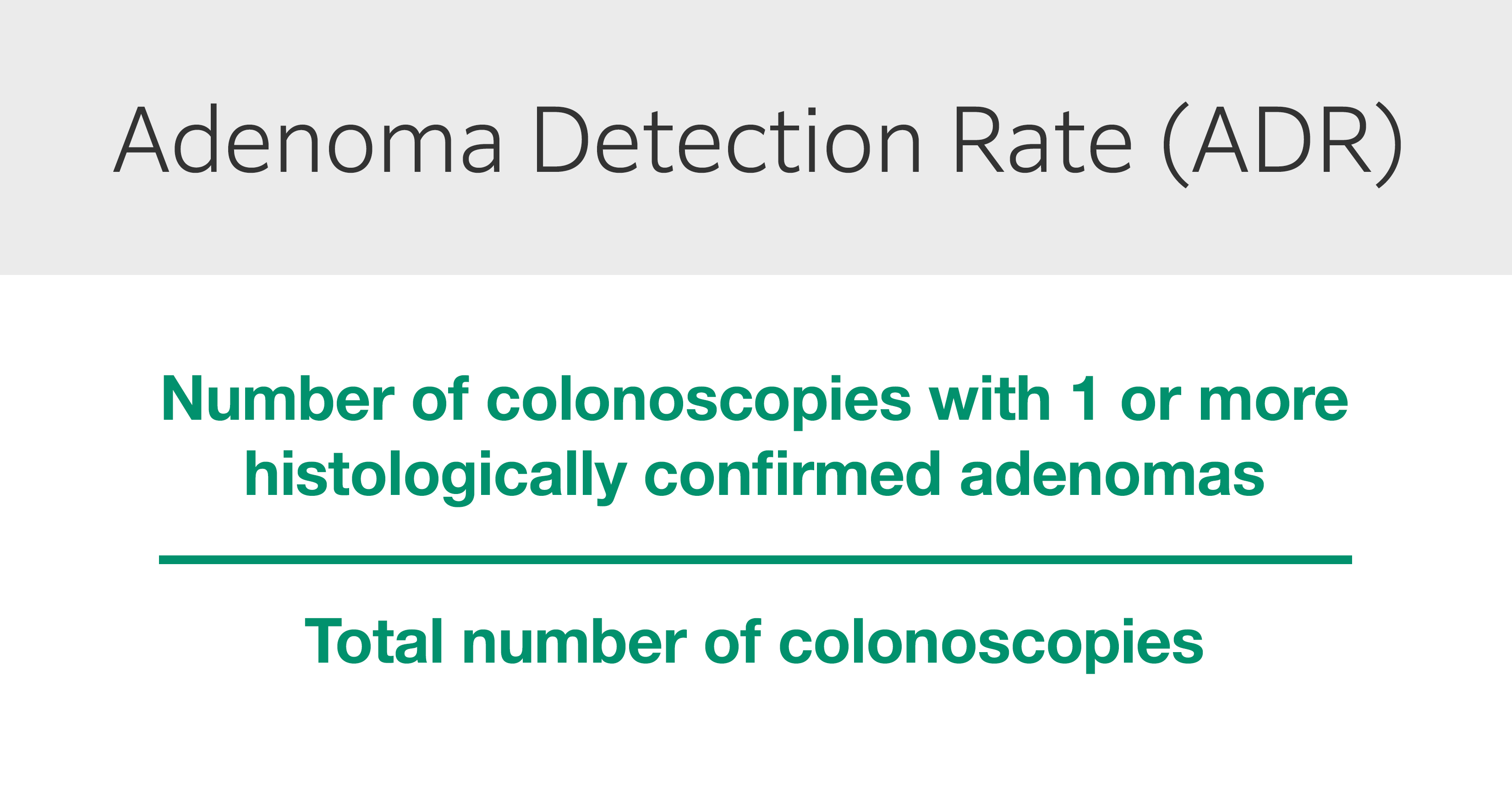
The adenoma detection rate (ADR) is a validated, independent predictor of interval CRC. First proposed by the US Multi-Society Task Force on Colorectal Cancer in 2002, ADR is defined as the percentage of colonoscopies at which one or more histologically confirmed adenomas is found and removed during a normal screening procedure for patients > 50 years old (Fig. 3).
The American Society for Gastrointestinal Endoscopy (ASGE) Taskforce CRC screening recommendations outline that ADR should be 25% overall or 30% for male patients and 20% for female patients, while the European Society of Gastrointestinal Endoscopy (ESGE) benchmarks call for an ADR of at least 25%.
The ADR has been widely accepted as a measure of colonoscopy quality for approximately a decade, but it is not without limitations. Evidence of high variability across practices suggests that careful review of ADR exclusion criteria is needed. For instance, starting measurement at 45 years — in response to the US guidelines update — shows minimal impact on ADR and would not require adjustments to the recommended threshold. Combined ADR (surveillance, screening, and diagnostic) is comparable to screening ADR, suggesting that expanding indications to include surveillance examinations in ADR calculations could streamline quality measurement implementation and reporting.

“In spite of us knowing we have conducted a good exam, we are still missing adenomas as a community“
Dr Sravanthi Parasa, MD, FASGE
sheds light on enhancing detection and exploring alternative quality measures.
But ADR refinement may not be enough: adenomas and advanced adenomas are still missed, and more frequently than previously believed, implying that ADR may be necessary but not sufficient to evaluate endoscopist performance. The “one and done” approach — by which the operator may be less inclined to examine the remaining colon after detecting one adenoma — is an inherent weakness of ADR calculations that can indirectly affect procedure quality without impacting the rate.
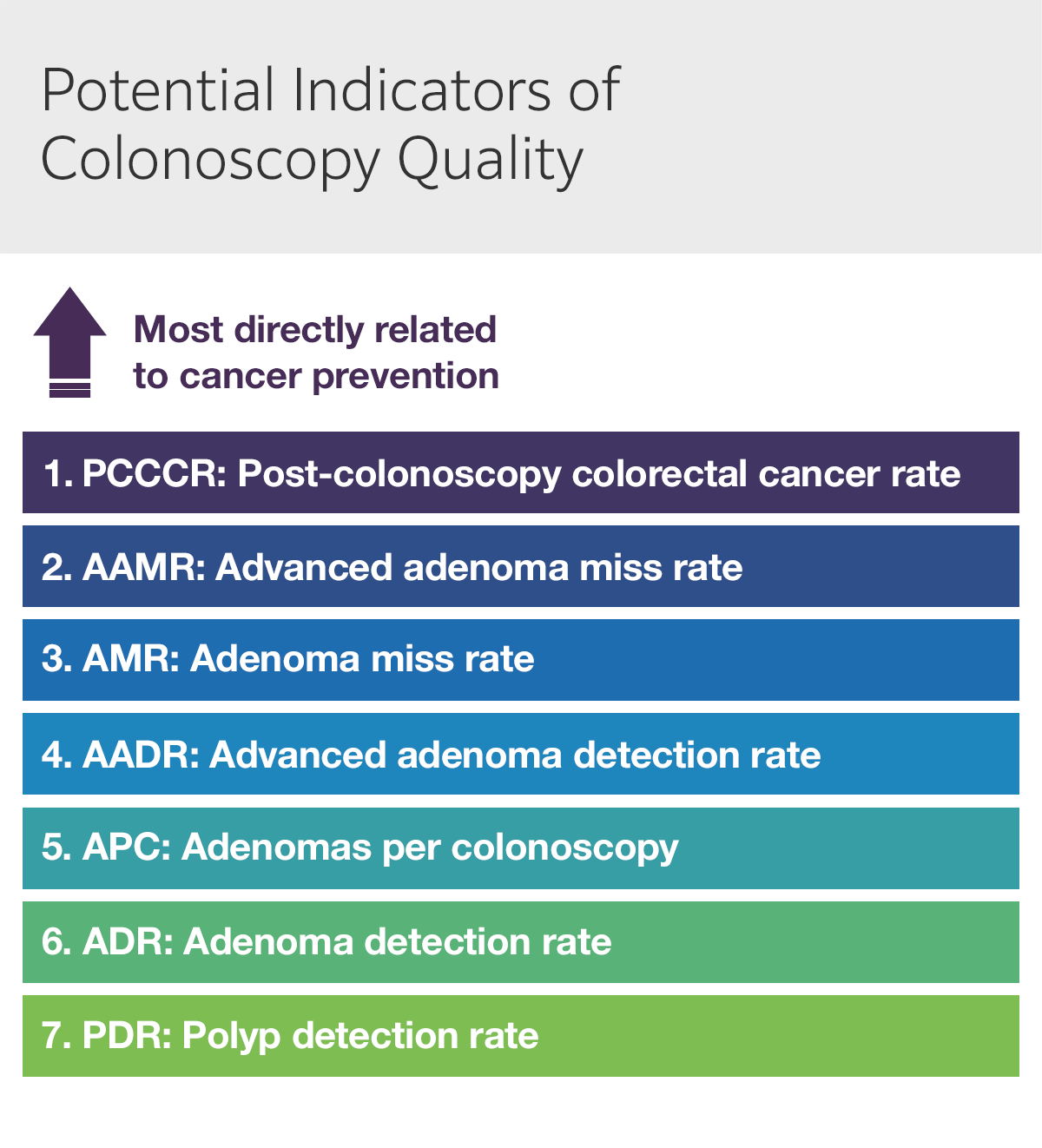
Several indicators have been proposed as detection measures for conventional adenomas based on their relationship with CRC prevention (Fig. 4):
- The adenoma miss rate (AMR) — and advanced adenoma miss rate (AAMR) — represent the ability to quantify total adenomas and advanced adenomas and are among the most accurate measures of colonoscopy quality. However, these rates are best estimated by back-to-back colonoscopy, difficult to apply in clinical practice. Furthermore, AMR is significant — and independent from ADR — even in quality-adjusted, back-to-back colonoscopies, although novel endoscopy systems may help address this issue. An optimal surrogate indicator of AMR should enable simple estimation and derive reliable results from a single, qualified colonoscopy.
- Adenomas per colonoscopy (APC) — or one of its variants, adenomas per index colonoscopy (APIC) and adenomas per positive index colonoscopy (APPC) — deserves careful consideration APC incentivizes full and complete clearing during colonoscopy, increasing overall detection rates and serving as an independent predictor of AMR. APC also offers additional information about endoscopist performance: although further studies are needed, available data suggests that an APC of ~0.6 correlates with current minimum ADR thresholds. On the other hand, APC presents important practical disadvantages such as increased costs due to sedation, withdrawal time, biopsy, and histopathology; however, natural language processing and real-time optical biopsy of polyps (with or without artificial intelligence) could render APC as a more feasible approach in the future.
Increased availability of newer technologies continues to reduce operator dependence and variability, improving quality regardless of the metric used — although ADR may eventually be rendered inadequate. Beyond their role in quality assessment, ADR, AMR/AAMR, and APC can also impact recommendations for surveillance intervals, underscoring the need for technological innovation that can ensure success.
References
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, et al. 2019. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 144(8):1941-1953, PMID: 30350310.
Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, et al. 2020. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 159(1):335-349.e15, PMID: 32247694.
Liang J, Jiang Y, Abboud Y, Gaddam S. 2022. Role of Endoscopy in Management of Upper Gastrointestinal Cancers. Diseases 11(1):3, PMID: 36648868.
US Cancer Statistics Working Group. US Cancer Statistics Data Visualizations Tool. [Website]. US Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. https://www.cdc.gov/cancer/dataviz (July 2025).
Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. 2023. Colorectal cancer statistics. CA Cancer J Clin 73(3):233-254, PMID: 36856579.
US Preventive Services Task Force. 2021. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 325(19):1965–1977, PMID: 34003218.
Knudsen AB, Trentham-Dietz A, Kim JJ, Mandelblatt JS, Meza R, et al. 2023. Estimated US cancer deaths prevented with increased use of lung, colorectal, breast, and cervical cancer screening. JAMA Netw Open 6(11):e2344698, PMID: 37991759. Erratum in: JAMA Netw Open 6(12):e2351369, PMID: 38113047.
Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, et al. 2014. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut 63(6):949-56, PMID: 23793224.
Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. 2017. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 112(7):1016-1030, PMID: 28555630.
Wang HS, Pisegna J, Modi R, Liang LJ, Atia M, et al. 2013. Adenoma detection rate is necessary but insufficient for distinguishing high versus low endoscopist performance. Gastrointest Endosc 77(1):71-8, PMID: 23261096.
Zhao S, Wang S, Pan P, Xia T, Chang X, et al. 2019. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: A systematic review and meta-analysis. Gastroenterology 156(6):1661-1674.e11, PMID: 30738046.
Rex DK. 2020. Detection measures for colonoscopy: Considerations on the Adenoma Detection Rate, recommended detection thresholds, withdrawal times, and potential updates to measures. J Clin Gastroenterol 54(2):130-135, PMID: 31851104.
Matyja M, Pasternak A, Szura M, Wysocki M, Pędziwiatr M, Rembiasz K. 2019. How to improve the adenoma detection rate in colorectal cancer screening? Clinical factors and technological advancements. Arch Med Sci 15(2):424-433, PMID: 30899296.

